About REACH
What is the REACH study?
REACH (Reversing the Epidemic in Africa with Choices in HIV prevention), or MTN-034, was a Phase IIa open-label study designed to fill important gaps in information about the safety and acceptability of the monthly dapivirine vaginal ring and Truvada® as daily oral pre-exposure prophylaxis (PrEP) in girls younger than 18 and to supplement existing safety and acceptability data of these products in young women ages 18-21. Both the ring and oral PrEP must be used consistently to prevent HIV – for the ring, a full month at a time, and daily for oral PrEP– which previous studies of these products found to be especially challenging for adolescents and younger women. As such, REACH also sought to understand what kind of support adolescent girls and young women need to use the ring and oral PrEP as best they can and their preferences for each. The study, which took place between February 2019 and September 2021, enrolled 247participants ages 16-21 who were assigned female at birth, at four sites in South Africa, Uganda and Zimbabwe, 86 or whom were under the age of 18 at the time of enrollment.
Who conducted and funded the study?
REACH was conducted by the Microbicide Trials Network (MTN), which from 2006 until November 30, 2021, was funded as an HIV/AIDS clinical trials network by the National Institute of Allergy and Infectious Diseases (NIAID), the Eunice Kennedy Shriver National Institute of Child Health and Human Development and National Institute of Mental Health, all components of the U.S. National Institutes of Health. Leading the study were Gonasagrie (Lulu) Nair, MBChB, MPH, of Stellenbosch University in South Africa, who was protocol chair, alongside Connie Celum, MD, MPH, of the University of Washington, USA, and Kenneth Ngure, PhD, MPH, of Jomo Kenyatta University of Agriculture and Technology in Kenya, serving as protocol co-chairs.
Where was REACH conducted?
REACH was conducted at four MTN-affiliated clinical research sites (CRSs): the Makerere University-Johns Hopkins University (MU-JHU) CRS in Kampala, Uganda; the University of Zimbabwe Clinical Trials Research Centre Spilhaus CRS in Harare; and, in South Africa, at the Wits Reproductive Health and HIV Institute in Johannesburg, and at the Emavundleni CRS of the Desmond Tutu HIV Foundation in Cape Town.
Why REACH?
According to UNAIDS, in 2020, one in four new HIV diagnoses in sub-Saharan Africa were among young women ages 15-24, even though they make up only 10 percent of the population. Ensuring that young women and girls can have access to a range of safe and effective HIV prevention methods is vitally important, because as has been seen with contraceptives, the more options that are available the more likely there will be one that can and will be used.
Daily oral PrEP is approved in many countries but it’s only one method, and for many adolescent girls and young women, it’s not a method they find easy or desirable to use. The dapivirine ring, which is the first biomedical HIV prevention product designed specifically for women and the first long-acting method, received a positive scientific opinion from the European Medicines Agency (EMA) in 2020 for its use in developing countries among women at high risk for HIV who cannot or choose not to use daily oral PrEP. Soon after, the World Health Organization (WHO) recommended the ring as an additional prevention choice for women. The ring’s developer, the nonprofit International Partnership for Microbicides (IPM), is seeking its approval in eastern and southern Africa, with approvals received in Zimbabwe and several other countries, and additional regulatory reviews underway. Because the Phase III trials of the ring were conducted among women ages 18-45, additional data, particularly on its safety, will be needed to support the ring’s use in women younger than 18. Data from REACH, which IPM intends to submit to both the EMA and African regulators, will be key.
How was REACH designed?
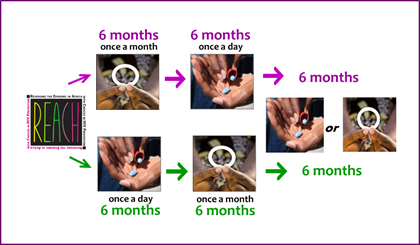 REACH was the first HIV prevention study incorporating the concept of informed choice into its design. In REACH, all participants used both the dapivirine ring and Truvada as daily oral PrEP, each for six months, with the order of use determined by randomization. After having experienced both products, participants were able to choose which one they wanted to use for the remaining six months of the study, or to use neither, and could change their minds. Throughout the study, participants received support and counseling focused on helping them to use their assigned or chosen product as best they could.
REACH was the first HIV prevention study incorporating the concept of informed choice into its design. In REACH, all participants used both the dapivirine ring and Truvada as daily oral PrEP, each for six months, with the order of use determined by randomization. After having experienced both products, participants were able to choose which one they wanted to use for the remaining six months of the study, or to use neither, and could change their minds. Throughout the study, participants received support and counseling focused on helping them to use their assigned or chosen product as best they could.
To evaluate the safety of each product, researchers conducted medical exams and laboratory tests of blood, urine and vaginal fluid. To evaluate adherence and acceptability, participants answered questions about their use and experience with each product, both on a computer and in face-to-face conversations with site staff. Some participants also took part in in-depth interviews or focus group discussions to help researchers gain additional insight into what motivated or was challenging about using each product. Laboratory tests for adherence were used to determine how well participants used each product. Adherence to oral PrEP was based on levels of drug in blood samples taken at each monthly visit. For the ring, researchers looked at the amount of residual drug left in rings participants return after a month of use.
What have we learned from REACH so far?
Results from the first two periods of the study, when participants used the dapivirine ring and oral PrEP for six months each, found both products to be well tolerated with no safety concerns, including in girls ages 16 and 17, data which was reported at the 11th IAS Conference on HIV Science (IAS 2021).
REACH has also shown that adolescent girls and young women can and will use the dapivirine ring and daily oral PrEP ring consistently, with results from the first two periods (also reported at IAS 2021) showing that the vast majority (97 percent) of the study’s 247 participants had used the vaginal ring or daily oral PrEP some or all of the time, and that fewer than three percent used neither of the products. Likewise, data reported in February 2022 at the Conference on Retroviruses and Opportunistic Infections (CROI) from the study’s the third and final period, when participants could choose between using the ring or oral PrEP, or neither, found nearly all participants had used their preferred method some or all of the time. Of the 247 participants who had enrolled in REACHs, 227 took part in the choice period.
Moreover, REACH has demonstrated the importance of providing adolescent girls and young women with options and letting them make their own decisions about which method is best for them. When asked which product they wanted to use during the final six months of the study, 152 participants (67 percent) chose the ring, 71 (31 percent) chose oral PrEP and only four participants (2 percent) opted to use neither. (Thirty switched products or changed their minds at least once during this time.). Among other things, these results suggest the monthly dapivirine ring could be a viable option for those adolescent girls and young women who can’t or choose not to use daily oral PrEP.
What were the reasons participants chose or switched from one product to another, or chose neither?
We know from data collected during the first two periods of the study that both approaches received high marks from participants, with 88 percent of participants saying they liked using the ring and 64 percent saying they liked daily oral PrEP. A detailed analysis of the study’s qualitative data is underway, looking at the specific aspects of each product that were perceived as advantages or disadvantages, and why participants selected one product over the other during the choice period, as well as why some decided to switch or chose neither. These results are expected to be reported mid-2022.
What did earlier trials of oral PrEP and the ring show in terms of younger women’s use of these products?
In the two Phase III trials of the ring, younger women used the ring least regularly, and as a group, had the lowest rates of risk reduction. In ASPIRE, for example, the ring was not shown to be effective among women ages 18-21, with levels of drug in returned rings also indicating low adherence. Likewise, daily pill taking was more challenging for younger women in the Phase III trials of oral PrEP (VOICE and FEM-PrEP). In the HPTN 082 open-label study among adolescent girls and young women in South Africa and Zimbabwe, found that 85 percent of the participants used oral PrEP with some regularity at the beginning of the study, but when clinic visits changed from monthly to every three months, there was a steep decline. Three months into the study, fewer than 25 percent were using oral PrEP, and by month 12, it was only nine percent, according to biological measures of adherence.
Why do you think REACH participants used the ring and oral PrEP so well?
The research team attributes the study’s findings of high product adherence and acceptability to the ongoing support measures, tailored specifically for each individual, and nonjudgmental counseling approach that focused on helping them to use their assigned or chosen product as best they could while also leaving the decision-making to them.
What happens now?
The study team is planning to publish detailed analyses of the study’s primary results, including data that have not yet been reported, in a series of peer-reviewed manuscripts. Primary results from the first two study periods are expected to be published mid-2022, with results from the choice period anticipated shortly thereafter. Meanwhile, IPM intends to submit data from REACH, as well as from the MTN-023/IPM 030 study among adolescent girls ages 15 to 17 in the United States, to both the EMA and to African regulators so that they may consider expanding the ring’s use to include adolescent girls where the product is approved. For its part, the WHO has already expressed interest in what insight REACH may provide for helping to better understand how best to support consistent and persistent use of both oral PrEP and the ring in adolescent girls and young women.
At the Trial Site
What kind of approvals were required to conduct this study?
REACH underwent extensive and rigorous review by NIAID and the US Food and Drug Administration. Moreover, before any site could begin enrolling women into the study, approvals were required of national regulatory authorities in the trial site country and by the site’s Institutional Review Board (IRB) or Ethics Committee (EC). IRBs and ECs ensure that studies are scientifically valid and ethically sound and provide oversight throughout the duration of the trial.
Did participants provide informed consent?
The legal age at which an individual may provide informed consent to enroll in a research study is 18. As such, in REACH, a participant under the age of 18 could only provide informed assent (agreement) with the permission of her parent and/or legal guardian also being required. Participants who turned 18 during the study were re-consented as adults. A participant who was under the age of 18 but considered to be an emancipated minor (because they are married, a mother or head of a household) could legally provide consent. Regardless, all participants who volunteered to join REACH (and their parents and/or guardians, if they were minors) were informed about all the study procedures, any possible risks, benefits and alternatives to participation, as well as the study’s requirements. Study staff also explained that they did not have to take part in the study and may leave it at any time, without consequence. All information was provided in simple terms and translated into local languages.
Who could enroll in REACH?
To enroll in REACH, women must have been between 16 and 21 years old, HIV-negative, have been using an effective form of contraception for at least two months before starting the study, and agree to use a contraceptive throughout participation. Potential participants who weren’t already using a contraceptive were counseled by site staff on different options and provided their method of choice prior to enrollment.
Wasn’t REACH supposed to enroll 300 participants?
REACH was originally designed to enroll 300 participants, including 100 between 16 and 17 years of age. However, in March 2020, in the face of the emerging COVID-19 pandemic, MTN and study leadership decided to close the study to further enrollment so that fullest attention could be paid to ensuring the safety of its current participants as well as clinic staff. By this time, REACH had already enrolled 247 participants, including 86 (35 percent) who were under the age of 18, such that the study would still be able to provide sufficient data about the safety of the dapivirine vaginal ring and oral PrEP in adolescent girls and young women, and to do so in less time as well.
Why were participants required to use contraception for at least two months before they could enroll?
Participants were required to be using a contraceptive for at least two months prior to enrollment to allow enough time for initial side effects to subside before beginning use of the dapivirine ring or oral PrEP. In this way, researchers – and participants – would be more certain that any side effects or changes that occur during product use were due to the ring or PrEP and not the contraceptive.
What did participation in the study involve?
Participants were in the study for about 19 months, with monthly clinic visits throughout. Each study visit included a meeting with a counselor to discuss their experience using the dapivirine ring or oral PrEP and strategies that might help improve their use. Participants also answered questions about their use and experience using the products on a computer. Some were asked to take part in in-depth interviews and focus group discussions to help researchers better understand what was motivating or challenging about using each product. To monitor the health and safety of participants, various tests and exams were conducted. Visits also included HIV testing and risk-reduction counseling, including the provision of condoms; testing and treatment of other sexually transmitted infections (STIs); and pregnancy testing and contraceptive counseling. At some visits, physical and pelvic exams were conducted.
How were participants supported to use the ring and oral PrEP?
As part of REACH, study participants received ongoing support and counseling focused on helping them to use their assigned (or chosen) product as best they could. Every monthly visit included a meeting with a counselor, and participants could also choose from a menu of additional forms of support, including daily text messages or weekly check-ins by phone, having a “Peer Buddy” and adherence support groups. At some sessions, participants received their individual adherence test results as a way to help facilitate discussion about adherence and how it relates to HIV risk reduction. Results were presented in terms of the participant’s likely level of HIV prevention – either low, medium or high – and without judgement. No matter what the results suggested, counselors provided encouragement and offered to help in whatever way they could. Study participants were also encouraged to be open with study staff about any concerns or difficulties they may have. If participants weren’t willing or able to use the ring or oral PrEP, researchers wanted to understand why so that other measures of support might be considered. At the same time, the study intended to help participants recognize that these products won’t be for everyone, and the reasons why were just as important for researchers to understand.
What measures were taken to ensure the safety of study participants?
REACH was conducted with numerous measures to protect the safety and wellbeing of participants. First, potential volunteers were carefully screened by site teams to ensure that only those for whom it would be safe to participate were enrolled. During the study, thorough evaluations of the health, safety and welfare of participants were conducted at regular intervals. In addition to these protections at the site level, participant safety was also monitored by MTN’s Statistical and Data Management Center, which assessed incoming reports daily, and by a Protocol Safety Review Team (PSRT) that met monthly. A Study Monitoring Committee (SMC) was responsible for general study oversight and conducted periodic reviews of study conduct, including participant safety. Based on its review the SMC could at any time recommend that the study proceed as designed, proceed with design modifications, or be discontinued.
Were there measures to address or protect against social harms and intimate partner violence?
Intimate partner violence is an unfortunate reality for many adolescent girls and young women in the communities where REACH was conducted. As such, the study team recognized that social harms or intimate partner violence could be experienced by some study participants. Participants could also experience social harms simply as a result of being in the study, or because they were using oral PrEP or a vaginal ring. They may have felt undue pressure from partners, family members or friends, or experienced stigma or discrimination from family members and members of their community. All sites had processes in place to address any instance of intimate partner violence or social harms to ensure participants were provided appropriate support and counseling by site staff and referred to any services not available at the site, and that measures were taken to protect her safety. Moreover, all sites were required to have a social harms committee with members who have expertise in areas such as research ethics, protection of minors, women’s rights law and gender-based violence response and prevention to ensure adequate support was provided to any participant who experienced social harm. The committee provided advice and guidance on possible referral organizations, legal obligations, and any other relevant resource or information with the goal of supporting participant autonomy and wellbeing while ensuring ethical obligations of the study. The study management team also provided ongoing support and problem solving for sites to provide comprehensive, trauma-informed care to participants.
What if a participant became pregnant?
While participants must use contraception while in the study, as precaution, pregnancy tests were conducted at each monthly clinic visit. Participants who became pregnant during the study stopped use of the ring or oral PrEP but remained in the study, continuing with routine study visits. These participants were referred by study staff to available sources of medical care and other relevant services Participants who completed pregnancy and breastfeeding while still in follow-up were able to resume use of the ring and/or oral PrEP.
What happened if a participant acquired HIV during the study?
A comprehensive HIV prevention package was provided to all participants throughout the trial. This includes HIV risk-reduction counseling, free condoms, regular HIV testing and testing for and treatment of other STIs. Despite these intensive efforts, a small number of participants acquired HIV during their participation in REACH. Participants who tested positive for HIV immediately stopped using their assigned or chosen product – the dapivirine vaginal ring or Truvada as PrEP – and received counseling and referrals to local HIV care and support services. These participants were encouraged to remain in the study and continue with routine study visits and mainly chose to do so.
About the Dapivirine Ring and Oral PrEP
What exactly is the dapivirine vaginal ring?
The dapivirine ring is the first biomedical HIV prevention product designed specifically for women. The ring is made of a flexible silicone material containing 25mg of the ARV drug dapivirine, about 4mg of which is released into the vagina when used continuously for about one month with low absorption elsewhere in the body. Women can insert and replace the ring themselves each month. IPM, the ring’s developer, is seeking its regulatory approval in several African countries.
What is the regulatory status of the ring?
In July 2020, the ring received a positive opinion from the European Medicines Agency (EMA) for its use among women ages 18 and older in developing countries, an important first step toward its potential approval by regulatory authorities in countries where women are especially vulnerable to HIV. Soon after, the ring was added to the World Health Organization’s (WHO) list of pre-qualified medicines. IPM is seeking approval of the ring in eastern and southern Africa, including in countries where REACH was conducted. In July 2021, the ring received its first approval, from the Medicines Control Authority of Zimbabwe. In anticipation of the ring’s potential approval, WHO’s updated guidelines for HIV prevention, published in March 2021, recommend the ring as an additional HIV prevention choice for women at substantial risk of HIV, while also acknowledging that studies like REACH will help to better understand ways to support consistent and persistent use of both PrEP and the ring in adolescent girls and young women.
What do we know about the dapivirine vaginal ring? Is it effective?
Multiple studies of the ring conducted in Africa, Europe and the US have shown it to be well-tolerated in HIV-negative women, with no safety concerns. The largest of these were two Phase III clinical trials – ASPIRE (MTN-020), conducted by the MTN, and The Ring Study (IPM 027), led by IPM – involving more than 4,500 women ages 18-45 in Malawi, South Africa, Uganda and Zimbabwe. Results, reported in 2016, found the dapivirine ring reduced the risk of HIV by approximately 30 percent overall. Higher levels of protection were seen in women who used the ring most regularly. Results of the HOPE (MTN-025) open-label extension (OLE) study for former ASPIRE participants, and the DREAM (IPM 032) OLE for former Ring Study participants, which were first reported in 2019, showed increased ring use and suggested a greater reduction in HIV risk (about 50 percent) than in the Phase III trials. Without a placebo group, however, these findings indicate a trend of increased HIV prevention with increased use, but don’t have the same strength of evidence.
What did the previous study of the ring in adolescent girls, MTN-023/IPM 030, find?
MTN-023/IPM 030 was a Phase IIa study that evaluated the safety and acceptability of the dapivirine vaginal ring among 96 girls between the ages of 15-17 at six sites in the United States. Results, which were reported in 2017, found no differences in safety outcomes between the dapivirine ring and the placebo ring. Adherence to the ring was also high, with 95 percent of the rings that participants returned to the clinic having drug levels suggesting regular use during the previous month.
What is oral PrEP?
Oral PrEP (pre-exposure prophylaxis), is an HIV prevention method that HIV-negative people can use to reduce their risk of HIV acquisition, that typically involves taking a daily pill. The ARV pill most commonly used as oral PrEP is Truvada, the brand name for a tablet containing the ARVs tenofovir disoproxil fumarate and emtricitabine that is marketed by Gilead Sciences of Foster City, California. Truvada was originally developed (and still used) for the treatment of HIV, in combination with other ARVs. Truvada was first approved for use as oral PrEP by the US FDA in 2012 and has since been approved in more than 50 countries.
In 2019, Gilead obtained FDA approval of a second drug for PrEP called Descovy®, which contains emtricitabine and tenofovir alafenamide (F/TAF). Approval does not apply to people at risk of getting HIV through receptive vaginal sex, because effectiveness in this population has not yet been evaluated. The safety and efficacy of F/TAF among cisgender adolescent girls and women will be evaluated as part of a trial Gilead is conducting in South Africa and Uganda.
# # #
Additional information about REACH can also be found at https://mtnstopshiv.org/reach-study and https://mtnstopshiv.org/news/studies/mtn034.
About the Microbicide Trials Network
The Microbicide Trials Network (MTN) was from 2006 until November30, 2021 an HIV/AIDS clinical trials network funded by the National Institute of Allergy and Infectious Diseases with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the U.S. National Institutes of Health. Based at Magee-Womens Research Institute and the University of Pittsburgh, the mission of the MTN was to conduct rigorous clinical trials designed specifically to support potential licensure of promising microbicides – products applied inside the vagina or rectum that are intended to prevent the sexual transmission of HIV. More information about the MTN, including studies pending results or are ongoing, is available at https://mtnstopshiv.org.
Click here for PDF version of this document.
15-February-2022
