Important note: Information on this page was accurate at the time of publication. This page is no longer being updated.
VOICE C and VOICE D Social and Behavioral Research Sub-studies of VOICE
Background and Context
- Women account for 60 percent of adults with HIV in sub-Saharan Africa, where unprotected heterosexual intercourse is primarily to blame for the region’s heavy HIV burden. Young women are especially vulnerable. Efforts to promote abstinence, monogamy and male condom use haven’t been enough to stop the epidemic nor are these methods feasible in most settings.
- Women urgently need safe and effective HIV prevention methods they can control themselves, and they must also be willing and able to actually use them. No matter how effective a given product or approach may be, it can have no benefit if it’s not used or not used correctly.
- The results of an HIV prevention trial called VOICE – Vaginal and Oral Interventions to Control the Epidemic – make this point all too clear: Despite living in communities severely impacted by HIV, most of the women in VOICE did not use the products being tested. Why is that? Two social and behavioral sub-studies of VOICE – VOICE C and VOICE D – hope to address some of the most pressing questions about VOICE, questions that have important implications for the success of future and ongoing HIV prevention efforts, as well.
This Questions and Answers document has three parts. General questions about VOICE and the study’s primary results are covered in the first part, followed by separate sets of questions for VOICE C and VOICE D.
I. VOICE: Vaginal and Oral Interventions to Control the Epidemic
1. What was the aim of the VOICE study?
VOICE – Vaginal and Oral Interventions to Control the Epidemic – was a major HIV prevention trial designed to evaluate whether antiretroviral (ARV) medicines commonly used to treat people with HIV are safe and effective for preventing sexual transmission of HIV in women. The study focused on two ARV-based approaches: daily use of an ARV tablet – an approach called oral pre-exposure prophylaxis, or PrEP; and daily use of a vaginal microbicide containing an ARV in gel form. Specifically, VOICE sought to determine the safety and effectiveness of three different products: an oral tablet containing tenofovir (known by the brand name Viread®); an oral tablet containing both tenofovir and emtricitabine (known as Truvada®); and tenofovir gel, a vaginal microbicide formulation of the oral tenofovir tablet. The study began enrolling women in September 2009 and completed follow-up of all participants in August 2012.
2. Where was VOICE conducted, and who participated?
VOICE, also known as MTN-003, was conducted at 15 clinical research sites in South Africa, Uganda and Zimbabwe, encompassing a region where HIV incidence is among the highest anywhere in the world. The study enrolled 5,029 sexually active HIV-negative women largely representative of the epidemic in sub-Saharan Africa; about half were under age 25, and most were not married.
3. Who conducted and funded the study?
As a flagship study of the Microbicide Trials Network (MTN), VOICE was funded by the National Institute of Allergy and Infectious Diseases (NIAID), with co-funding from the Eunice Kennedy Shriver Institute for Child Health and Human Development and the National Institute of Mental Health (NIMH), all components of the U.S. National Institutes of Health. It was led by Zvavahera Mike Chirenje, M.D., from the University of Zimbabwe in Harare; and Jeanne Marrazzo, M.D., M.P.H., from the University of Washington in Seattle. The study products were provided by Gilead Sciences, Inc., of Foster City, Calif., and by CONRAD, of Arlington, Va. Viread (oral tenofovir) and Truvada are registered trademarks of Gilead Sciences. In 2006, Gilead assigned a royalty-free license for tenofovir gel to CONRAD and the International Partnership for Microbicides of Silver Spring, Md.
4. How was VOICE designed?
Determining the safety and effectiveness of each approach (daily use of tenofovir tablets, Truvada tablets or tenofovir gel) required the kind of trial in which participants were randomly assigned to different study groups, including groups that used a placebo, which has no active drug. Moreover, because the study was “blinded,” neither participants nor researchers knew who was in which group while the study was ongoing. Like all HIV prevention trials, women in VOICE received ongoing HIV risk reduction counseling, condoms and diagnosis and treatment of sexually transmitted infections – standard measures for preventing HIV – throughout their participation.
VOICE originally had five study groups (also called arms) – two gel groups (tenofovir gel and an 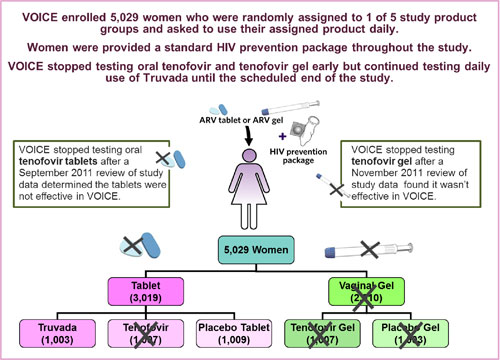 inactive placebo gel) and three tablet groups (tenofovir, Truvada and an inactive placebo tablet) – with about 1,000 women in each group who were instructed to use their assigned study product every day. In late 2011, VOICE stopped testing oral tenofovir and tenofovir gel, however, after separate routine reviews of study data by the trial’s independent data safety and monitoring board (DSMB) determined that while each was safe, neither was effective in preventing HIV compared to its matched placebo. VOICE continued to evaluate Truvada until the scheduled end of the study.
inactive placebo gel) and three tablet groups (tenofovir, Truvada and an inactive placebo tablet) – with about 1,000 women in each group who were instructed to use their assigned study product every day. In late 2011, VOICE stopped testing oral tenofovir and tenofovir gel, however, after separate routine reviews of study data by the trial’s independent data safety and monitoring board (DSMB) determined that while each was safe, neither was effective in preventing HIV compared to its matched placebo. VOICE continued to evaluate Truvada until the scheduled end of the study.
5. What are the results of VOICE?
VOICE found none of the three products effective in preventing HIV among the women in the study. Moreover, the study’s results indicate that most participants had not used their assigned product daily as recommended. Younger, unmarried women were least likely to use study product, and they were also most likely to acquire HIV, with HIV incidence in this group approaching 10 percent at some study sites in South Africa, a rate considerably higher than expected. HIV incidence, which reflects the number of women who become newly infected for every 100 participants in a given year, was 5.7 percent overall, double what the researchers had anticipated it would be. Taken together, these findings suggest that daily use of a product – whether a vaginal gel or an oral tablet – was not the right HIV prevention approach for the women in VOICE. No safety concerns were identified. These results were first reported at the Conference on Retroviruses and Opportunistic Infections in March 2013 (see Understanding the Results of VOICE). A more detailed analysis of VOICE data, when available, will help in better understanding these results as well as answer other questions VOICE was designed to address.
6. How do you know women didn’t use the study products?
At the end of the study, an analysis of stored blood samples from a subset of 773 participants who had received the active products (including 185 women who acquired HIV) found adherence to product use was low across all groups. Drug was detected in less than a third of blood samples from women who were assigned to use either Truvada or oral tenofovir tablets and in less than a quarter of samples from women asked to use tenofovir gel. In sharp contrast, adherence was estimated to be about 90 percent based on self-report measures and monthly counts of unused gel applicators and leftover pills.
Researchers are now analyzing every sample collected from all 5,029 participants in VOICE – more than 160,000 plasma samples alone – to better understand the relationship between product use and product efficacy. The results of these analyses, expected sometime in 2014, are not likely to change the study’s main conclusions, however.
7. What results are most concerning to VOICE researchers?
Of great to concern to VOICE researchers were the study’s findings highlighting the gravity of the epidemic in a population that continues to be among the most vulnerable: young, single women. HIV incidence was 8.8 percent for unmarried women younger than 25, more than 10 times higher than the HIV incidence or the older, married women in VOICE, which was less than 1 percent. Moreover, young, single women were much less likely to use their assigned study product. In the Truvada group, for example, drug was detected in the blood of just 21 percent of younger, single women compared to 54 percent of those married and over age 25.
8. Were participants in VOICE counseled about the importance of adherence?
Participants were counseled at each visit about the importance of adhering to the study regimens, product use and safe sex practices. Moreover, participants were informed of results of other studies that found the same products effective when used consistently, such as CAPRISA 004, which tested tenofovir gel used before and after sex, and the Partners PrEP Study, which evaluated daily use of Truvada and tenofovir tablets.
9. Why didn’t these high-risk women use the study products in VOICE?
This is one of the most important questions being asked about the results of VOICE, and one of many that are critically relevant to the entire field of HIV prevention. Understanding the impediments to adherence will be important to the success of future and ongoing efforts as well as for “real-world” use of products now being tested in clinical trials. The women who need safe and effective HIV prevention methods must also be willing and able to use them. Two social and behavioral research sub-studies of VOICE – VOICE C and VOICE D – hope to address some of the most important questions about VOICE, including why these high-risk women didn’t use the study products.
10. What do you expect to learn from VOICE C and VOICE D? Why are these sub-studies important?
Absent a “gold standard” for measuring adherence, researchers have relied on multiple methods in hopes of being able to piece together as accurate a picture as possible about participants’ study product use during a trial. But, what does it mean when one measure suggests high adherence but another shows just the opposite, as in VOICE? Taken alone or in combination, current measures of adherence are not telling the full story.
As such, VOICE C and VOICE D may be especially important for helping to make sense of the disparate findings in VOICE, by providing greater insight about women’s attitudes and behaviors and their reasons for using or not using the products. Moreover, both studies will help in understanding women’s social contexts, perceptions about HIV risk, their motivations for taking part in VOICE, and the deterrents to both product use and being open about these difficulties with study staff. What is learned in VOICE C and VOICE D may suggest ways that HIV prevention trials can glean more accurate and meaningful information about product adherence as well as help inform the development of products that women may find more practical and easier to use, and importantly, that they will actually use.
The first set of results from VOICE C was published in February 2014, while results from VOICE D are expected before the end of the year. Both sub-studies are funded by NIAID and NIMH of the U.S. National Institutes of Health.
11. After VOICE, what now? Are there other HIV prevention trials involving women?
There are currently three Phase III HIV prevention trials specifically focused on women. These are evaluating different strategies and new prevention approaches that researchers are hopeful will be easier for women to use than the daily regimens tested in VOICE. FACTS 001 is testing tenofovir gel used before and after sex and plans to enroll 2,900 women at nine South African sites, with results expected in 2015. As sister studies, ASPIRE, which is being conducted by the MTN, and The Ring Study, which is a trial of the International Partnership for Microbicides, are both assessing whether a vaginal ring containing the ARV drug dapivirine is safe and effective for protecting against HIV when used by women for a month at a time. Results of these two studies, which will involve nearly 5,000 African women, are expected late 2014 or early 2015.
12. Is more attention being paid to adherence to product use in these trials?
Certainly, the results of VOICE have caused current trials to reevaluate and/or strengthen their efforts to enhance product adherence, including helping current and prospective trial participants and local communities better understand the importance of correct and consistent product use and the impact that non-adherence can have on the findings of a research study. Many of these trials have already incorporated ways to better understand product adherence while the trial is underway so that the researchers can be made aware of and address challenges as they occur. In ASPIRE, for example, participant blood samples are being tested on a routine basis to determine the presence of active drug, but in a way that preserves the blinded, placebo-controlled nature of the study. So, while the study investigators and participants don’t know individual participant results, data pooled according to sites or the study overall could indicate a need to modify ongoing adherence counseling approaches, enrollment activities or community messages about ASPIRE.
When participants use the study product as directed in an HIV prevention trial, researchers can determine with greater certainty whether the product prevents HIV. At the same time, a finding of low adherence in the setting of a clinical trial, in which participants are constantly reminded to use the study product, may be indicative of the kinds of challenges women may face in the “real world.”
II. About VOICE C
13. What is VOICE C?
VOICE C, also known as MTN-003C or the Community and Adherence Sub-study, was conducted in parallel with VOICE and was designed to identify the specific factors and beliefs within women’s communities, social groups and households that may have influenced their willingness or ability to follow the daily regimens being tested in the main VOICE trial.
VOICE C was conducted at Wits Reproductive Health and HIV Institute (Wits RHI) in Johannesburg, South Africa, one of 15 clinical research sites for VOICE. Jonathan Stadler, Ph.D., of Wits RHI, and Ariane van der Straten, Ph.D., M.P.H., of RTI International/Women’s Global Health Imperative (WGHI) program in San Francisco, led the study.
14. Who participated in VOICE C?
VOICE C included 102 women enrolled in VOICE at WRHI, as well as 26 male partners, 17 members of WRHI’s Community Advisory Board (CAB) and 23 key community stakeholders, including AIDS activists, health care workers, church leaders, local journalists and local government officials, for a total of 164 participants. Everyone who participated in VOICE C provided written informed consent. For women in VOICE, a separate written consent was obtained for the VOICE-C sub-study.
15. How was VOICE C designed?
VOICE C involved the use of in-depth interviews, focus group discussions and ethnography, an approach that affords a closer look at the daily lives of people as well as the local surroundings and household dynamics that can shape different behaviors. VOICE C researchers hope that the combination of these different approaches will allow them to piece together the most salient issues, identify discrepancies or sources of misunderstandings and see where attitudes either converge or conflict to influence a women’s ability and willingness to use the gel or tablets.
The women in VOICE C were randomly assigned to take part in either a focus group discussion at the end of their participation in VOICE (with separate groups for those who used gel and for those assigned to take a tablet), a one-time individual in-depth interview while still participating in the trial, or in a series of two to four ethnographic interviews, which involved a researcher spending several hours at their homes, much of this time engaging in open-ended conversation.
Male partners, CAB members and community stakeholders were asked to participate in either in-depth interviews or focus groups. VOICE C researchers also conducted observations in the community, including at events promoting recruitment into VOICE.
16. What specific topics were covered by researchers in their interactions with the women in VOICE C?
Whether through a focus group discussion, in-depth interview or an ethnographic visit, researchers sought to understand women’s experience being in VOICE and in using their assigned study product, particularly, whether a daily regimen was difficult in any way. Researchers also asked women about their living quarters, relationships with different family members, family members’ HIV status, and about the women’s work or other activities outside the home. Of great interest to researchers was learning whether women’s partner(s) were aware of and approved their use of the study product and what friends, neighbors or others in the community may have felt about their being in a study testing ARV-based products as HIV prevention, since ARVs are normally used by people infected by HIV.
17. What do expect to learn from the male partners enrolled in VOICE C?
With the permission of the women participating in VOICE C, researchers obtained consent of 26 male partners; who took part in an in-depth interview or a focus group discussion either during or at the conclusion of their partner’s participation in VOICE. Questions and discussion with the men focused on their own and their partners’ experience with the study, and what they liked and disliked about the study and the tablets or gel that were used.
18. Why did VOICE C involve CAB members and community stakeholders?
By including both CAB members and key community stakeholders in VOICE C, researchers hope to gain better insight about the opinions and feelings of people who are more distant from the study or research site, the community’s overall acceptance and understanding of the trial and study-related rumors. Researchers hope also to gain better understanding of social norms around HIV prevention, cultural beliefs and traditions, and practices around marriage, gender, sexual relationships that may have influenced participants’ adherence to either regimen.
19. How did the closure of the tenofovir gel and oral tenofovir arms in VOICE affect VOICE C?
When VOICE stopped testing oral tenofovir and tenofovir gel early, after independent reviews of study data determined that neither product was effective in preventing HIV compared to its matched placebo, women in the affected groups were told to stop taking their assigned study product. They were then followed for an additional eight weeks, the normal plan for all participants as they exit off the study. Closing the tenofovir tablet arm of VOICE meant that women and study staff would be “unblinded” and learn who had been assigned to use tenofovir tablets. Likewise, the decision to stop testing tenofovir gel meant that VOICE would also need to close the placebo gel arm of the study. At their last study visit, the women in the two gel groups were told whether or not they had been using an active product.
Some adjustments to VOICE C were required to accommodate the closure of these arms, particularly with respect to the timing of focus group discussions and ethnographic visits for the women who had been randomized to those groups in VOICE C. While the protocol for the sub-study called for focus group discussions to take place at the end of women’s participation in VOICE, for the women in the oral tenofovir, tenofovir gel and placebo gel arms, these had to be scheduled within the eight-week window of their exiting the study. As such, focus group discussions involving these women were conducted in late 2011 and early 2012. Focus group discussions for the women in the Truvada and oral placebo study groups took place at the end of the trial, as originally planned.
Women who were to take part in the series of ethnographic visits who already had participated in at least one of these visits, took part in one more before having to exit the study. Women who had not yet had an ethnographic visit at the time they stopped using the product, took part instead in a single in-depth interview soon after being notified that the arm had closed.
20. Have researchers reported any results yet?
Researchers reported the first set of results 21 February, 2014 in the online journal PLOS ONE, which involved the group of VOICE participants. According to the findings, most women claimed they were able to use their assigned products but they alleged other participants were not. All but two women mentioned knowing or hearing about other participants who were not following the study’s regimens, often times while sitting in the clinic’s waiting room. The findings suggest that some of the reasons for women not using the study products included ambivalence about participating in a blinded clinical trial in which it wasn’t known whether they had been assigned to use an active product or a placebo, or that the active products were even effective; worries about both the side-effects and the stigma associated with the use of products meant for people infected with HIV; and pressure from loved ones or strains on relationships with partners, family and friends.
21. When will other results of VOICE C be available?
The research team is currently analyzing all the data collected from all study groups in VOICE C and anticipate reporting additional results later in 2014.
III. About VOICE D
22. What is VOICE D?
VOICE D, or MTN-003D, is an ongoing sub-study of VOICE that was launched in December 2012, after the main VOICE study was completed. VOICE D aims to better understand women’s actual and reported use of study products and sexual behavior during the time they took part in the VOICE trial. In particular, it seeks to understand why most women (4,578 of the 5,029 participants, or 91 percent) remained in VOICE yet so few adhered to product use (and were willing to admit non-use), despite living in communities with very high HIV incidence.
VOICE D complements and expands upon VOICE C by focusing more on women’s individual experiences, behaviors, beliefs and attitudes about HIV risk and ARV-based prevention, and in understanding the dynamic between trial participants and trial staff – all of which may have influenced whether or not products were used.
Its design intends to allow for more open discussion among former VOICE participants, especially among women who had reported using their assigned product but whose blood tests indicate otherwise.
VOICE D is being led by Ariane van Straten, Ph.D., M.P.H., of RTI /WGHI, with Barbara Mensch, Ph.D., of the Population Council and Elizabeth Montgomery, Ph.D., also of RTI/WGHI.
23. Where is VOICE D being conducted and who is participating?
VOICE D is being conducted at five of the 15 clinical research sites (CRSs) where VOICE took place: In Uganda, at the Makerere University-Johns Hopkins University Research Collaboration CRS in Kampala; in South Africa, at two CRSs (Overport and Isipingo) in KwaZulu-Natal affiliated with the Medical Research Medical Research Council in South Africa; and in Zimbabwe, at two CRSs affiliated with the University of Zimbabwe-University of California San Francisco (UZ-UCSF), the Seke South CRS in Harare and Zengeza CRS located Chitungwiza. In total, between 200 and 250 former VOICE participants will be enrolled in VOICE D.
24. What is the difference between VOICE C and VOICE D?
Unlike VOICE C, VOICE D was designed to be conducted after women completed their participation in VOICE, and it includes women from all three countries where VOICE was conducted. While it also employs in-depth interviews and focus groups discussions, an important difference is that in VOICE D these are conducted in a “neutral” location (away from the trial site) by researchers who had never interacted with participants during VOICE. These and other measures to “distance” VOICE D from the parent study are meant to encourage honesty and candid discussions about VOICE among its former participants, who may have been inclined to say what they expected clinic staff wanted to hear during their time in VOICE.
25. How is VOICE D designed?
VOICE D is being conducted in two phases. Phase 1, which has been completed, involved 88 women who took part in individual one-time in-depth interviews after exiting VOICE, and for 73 of these women, before the trial’s results were publicly reported and shared with participants and communities. Phase 1 was designed in part to better understand women’s perceptions and understanding of various risk behaviors, including anal sex.
Researchers added Phase 2 in response to VOICE results finding none of the three products (oral Truvada, oral tenofovir and vaginal tenofovir gel) was effective and that most women hadn’t used them. Phase 2 is enrolling former VOICE participants who had been assigned to use an active product during the trial and whose laboratory tests of stored blood – to detect the presence of active drug – indicate what their actual product use was. The goal is to enroll between 108 and 144 women, and to include HIV-negative “high adherers” (women who had drug present in most of their blood samples), HIV-negative “low adherers” (women who never or rarely had detectable drug in their blood samples), as well as women who acquired HIV during VOICE.
26. Why is VOICE D focusing on understanding anal sex practices of VOICE participants?
At enrollment, 17 percent of the women reported having had anal sex, and although questions about these behaviors were asked during VOICE, women may have misinterpreted their meaning or been afraid to respond honestly. Because unprotected anal sex is a major risk factor for HIV, it will be important to understand whether it was under-reported or over-reported in VOICE, especially in the context of a trial that found none of the products tested – including a vaginal gel – was effective against HIV.
27. What do researchers hope to gain by confronting women with information about their actual patterns of product use while in VOICE?
The majority of women in VOICE complied with the study’s monthly clinic visits as well as told study staff that they were using their assigned products. Yet, an analysis of stored blood samples from a subset of VOICE participants who had received the active products indicates that, in fact, most women were not using the products. Drug was detected in less than a third of blood samples from women who were assigned to use either Truvada or oral tenofovir tablets and in less than a quarter of samples from women asked to use tenofovir gel.
In VOICE D, the very data that is disconcerting to researchers will be used as a tool for understanding – from the women themselves – why the results are what they are.
The hope is that this information, along with results of participants’ individual blood tests, will encourage women to be forthcoming in individual interviews and/or focus group discussions and explain why they hadn’t (or hadn’t always) used their assigned product, or, for a minority of women, how they managed to use product consistently.
The study team realizes that some women may, for a variety of reasons, not be able to or want to talk about their actual product use. In a group, however, women may be more candid, especially about specific aspects of the trial’s procedures, counseling methods or their own social contexts and experiences that may have influenced behaviors, including how they communicated with study staff. For instance, did women feel compelled to use the gel or take tablets just before a study visit to please investigators, only to revert to nonuse afterward? When women enrolled in the trial, did they ever intend to use their assigned study product, or did they participate for other reasons, including for the health care services or HIV testing the study provided? If they had intended to use their assigned products, what prevented them from doing so?
These discussions may also provide insight for how current and future HIV prevention trials can collect more accurate and meaningful information about product adherence as well as understand the kinds of products women may find more practical and easier to use, and that they will actually use.
28. When are results of VOICE D expected?
VOICE D researchers expect to complete Phase II of the sub-study by the end of 2013 and report results of both phases of the study by mid-2014.
# # #
Additional information about VOICE and the VOICE C and VOICE D sub-studies can be found at http://www.mtnstopshiv.org/news/studies/mtn003.
About the Microbicide Trials Network
The Microbicide Trials Network (MTN) is an HIV/AIDS clinical trials network established in 2006 by the National Institute of Allergy and Infectious Diseases with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the U.S. National Institutes of Health. Based at Magee-Womens Research Institute and the University of Pittsburgh, the MTN brings together international investigators and community and industry partners whose work is focused on the development and rigorous evaluation of promising microbicides – products applied inside the vagina or rectum that are intended to prevent the sexual transmission of HIV – from the earliest phases of clinical study to large-scale trials that support potential licensure of these products for widespread use. More information about the MTN is available at www.mtnstopshiv.org.
Click here for PDF version of this document.
21-February-2014
See Also
MTN-003 (VOICE) Study Protocol
MTN-003C (VOICE C) Study Protocol
MTN-003D (VOICE D) Study Protocol
